The Weird and Wonderful Logic of Aluminium
Finally, an excuse to witness the one major metallurgical process I never saw when writing Material World.
One of the first challenges I faced when planning Material World was coming up with the list of substances I’d be covering. Needless to say, the initial list was not quite the same as the one I ended up with.
For one thing, the original plan was for seven materials, not six: the seventh substance was going to be wood. So I read an enormous pile of books on forest management and cultivation and actually spent some time out in woodland in Wales watching how 21st century forestry actually functions. None of that material ever made it into the book, because, as I began writing I realised that if I’d kept wood in there the whole thing would have been enormously long (and the book is pretty long as it is).
In the very initial drafts I assumed my battery material of choice would be cobalt rather than lithium. But the deeper I delved into the science and practice of batteries, the more I realised that while there are undoubtedly good journalistic reasons for focusing on cobalt (primarily the fact that most of it comes from the DRC and conditions there can be grim), in practice cobalt is becoming less important as different cobalt-free battery chemistries are growing far faster than cobalt-dependent chemistries. So lithium it was.
Another material I considered for a while was aluminium.
There is no doubting it is among the world’s most important substances. Without aluminium, a strong, light metal, we might never have mastered powered flight. Even today, when composites are becoming more prevalent in fuselages and wings, aluminium is still essential for aircraft manufacture. Then there’s our power systems: aluminium is the metal of choice for the high voltage power cables you see on pylons around the world (copper is more conductive but also far more heavy, so not best suited for stringing between tall towers). And, more and more these days, aluminium is being used in cars - especially electric cars where weight savings are all-important.
But I only had space in Material World for one or two industrial metals. Steel had to be there; so too did copper - so no aluminium. Long story short, I never got to go and visit an aluminium smelter, which was rather saddening. All of which is to say, when I had the opportunity to visit one for my day job at Sky News, I leapt at it.
Many years ago there were lots of aluminium smelters in the UK. There was one in Anglesey, another in Northumberland and a whole series of them in Scotland. Today there is only one left, the smelter at Lochaber, located just outside Fort William.
The Lochaber smelter is rather extraordinary, not merely because aluminium smelters are themselves rather extraordinary things. We take this metal for granted these days - it is workaday, humdrum, even. The other day I had to buy a new set of handlebars for my bike and I was struck that the aluminium option was the cheap, boring option, while carbon fibre or titanium were the far sexier alternatives.
But not long ago aluminium was a true wonder metal. Before the 19th century no-one had even set eyes on it; refining it was so hard that aluminium was the most precious of all metals. Napoleon III famously impressed his guests by serving them food not on plates of gold but of aluminium. The little aluminium block on the very top of the Washington Monument was, when it was put in place in 1884, the biggest piece of pure aluminium on the planet.
Two years later, the Hall–Héroult process was invented, making it possible for the first time to mass produce aluminium and the world changed forever. That probably sounds a little over the top, but it’s hard to conceive of how the Wright brothers could have lifted the Kitty Hawk flyer into the air without using aluminium as the metal for their engine. It’s hard to imagine how we could have built the thousands of miles of electrical grids we have today relying solely on copper. So working out how to make aluminium - not just in the laboratory but in vast quantities, was an extraordinary breakthrough.
Today, aluminium is still made using the Hall–Héroult process, in Lochaber and indeed elsewhere around the world. Alumina, a ground down sand of aluminium oxide (refined from bauxite, much of which is mined in Guinea and Australia) is poured into a cell, alongside molten cryolite - a kind of flux which lowers the melting temperature of the metal. Large carbon electrodes are lowered into it and a massive electrical current is run through it. Via this process of electrolysis, the oxygen atoms are wrenched off the alumina, and the molten, pure aluminium sinks to the bottom of the cell.
Having seen a few electric arc furnaces in my time - the electrical process whereby steel is made from scrap these days - the most striking thing about seeing the Hall–Héroult process in person is (and I realise this might sound odd) how quiet it is. Electric arc furnaces are so loud you can hardly hear yourself - which figures - the electrodes are creating a lightning storm (the arc) in their cauldron - but the cell room at Lochaber had at most a low bassy hum as the enormous electrical current did its business.
To get anywhere close to these cells you have to wear quite a lot of protective equipment. Not just the steel toecap, heat proof boots and the flame retardant clothes, but a full face helmet with a ventilator so you’re not breathing any of the air inside the room. Why? Because the chemical reaction inside those cells (especially the one between the cryolite and the alumina) generates all sorts of toxic gases you really don’t want to inhale.
Anyway, you can see the whole thing in the film I made for Sky. Cell room moment at about the 3:30 mark but do watch the whole thing if you have time. It’s the product of many weeks of work from me and a brilliant team at Sky, led by producer Aoife Yourell.
But here’s one nerdy detail that might appeal to anyone who has read Material World. What I hadn’t quite realised about aluminium is the defining feature of the cells you use for the Hall–Héroult process is that you can’t ever really turn them off. An electric arc furnace can be switched on and off at will (blast furnaces, as you will know from the book, are much harder to shut down). Anyway, the upshot is, when electricity is expensive or there’s no demand for steel, you just leave the arc furnaces off. You simply can’t do the same thing with aluminium smelters.
Have a look at these two charts below, both of which cover the same period (apologies for how shonky they look - I’m trying to do this on my iPad - a mistake, it transpires). On the left is US steel production, on the right US aluminium production each month going back to 2017. The key point here is that smack bang in the middle of each chart is the pandemic.
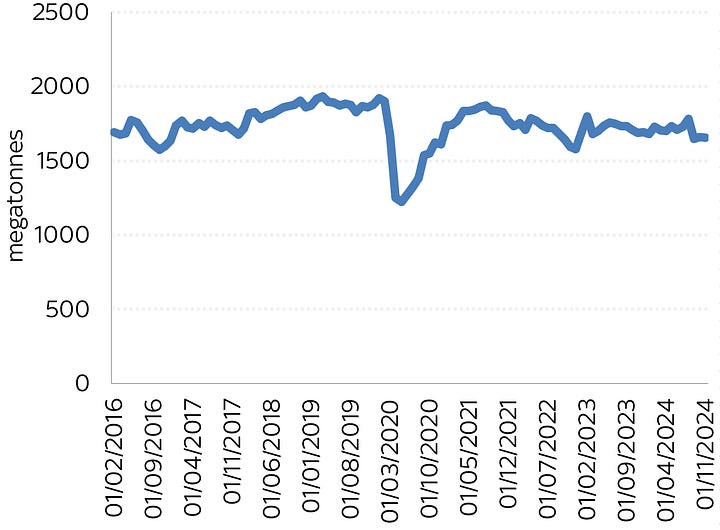
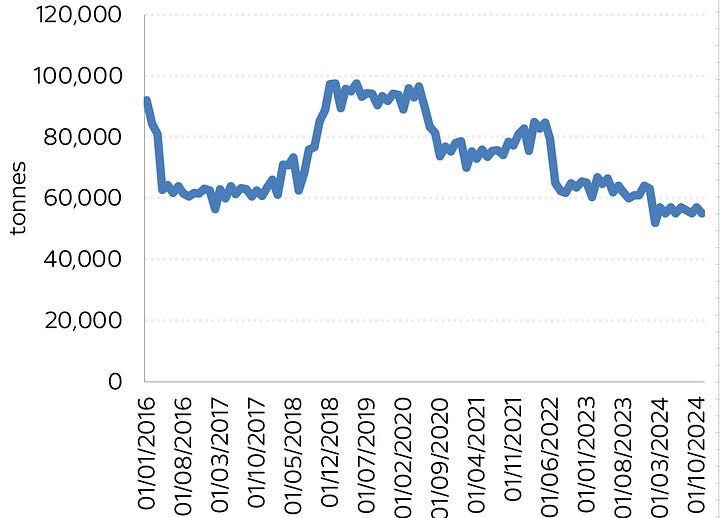
Can you spot the critical difference? In 2020, when lockdowns hit and global demand suddenly dropped, so too did US steel production. Why? The steelmakers simply turned off their electric arc furnaces. But look at aluminium - it never dropped during the pandemic. The line only drops when a smelter is mothballed permanently.
This might all seem like a small point, but it’s pretty massive. If you lose the power to an aluminium smelter then you have roughly four hours until the metal in those cells freezes and then the whole operation is dead. It’s a total disaster - the worse-case scenario for any aluminium plant. You’d have to rebuild the entire “pot line”, as they call the cells.
That helps explain why aluminium plants are always, always located near a reliable source of power. You cannot power an aluminium smelter with wind or solar. So historically they have been plugged into hydroelectric plants on very, very big rivers with a massive flow that will never dry out, or nuclear plants.
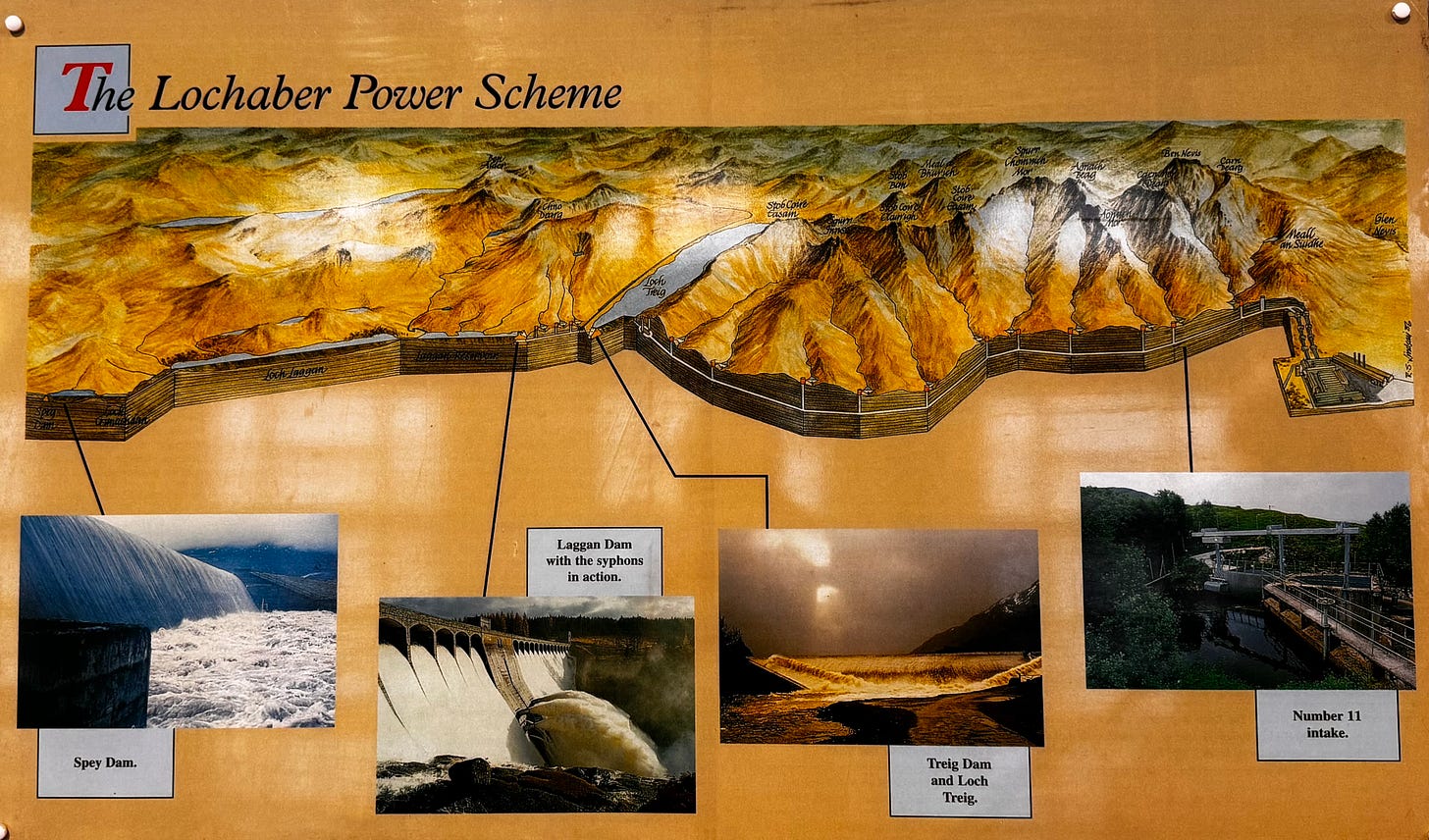
All of which brings us back to what make the Lochaber plant so interesting. Because while Scotland has plenty of rain, it doesn’t have the enormous roaring rivers of the kind you find in Canada or Russia. So the designers of this plant did something rather unusual. Instead of putting a dam in the way of a river and using it to create a current (as is the case for most hydro dams and most aluminium plants plugged into them) they created a complex series of dams and tunnels, all of which are designed to do one thing: to channel the rainwater from across a set of watersheds towards a single plant.
So they dug a long set of tunnels all the way through the flanks of Ben Nevis, connecting a whole series of different valleys. This was no mean feat. The tunnels were, at the time, the longest underground water tunnels in the world. It was a scheme the likes of which had never been seen anywhere else in the world. Go to one of these dams, the Laggan Dam, and you see a plaque commemorating the achievement.



Anyway, all those tunnels and dams are there for a single, simple reason: to ensure the water never, ever runs out - so the hydroelectric plant can always generate enough power to keep the cell room going, so the molten aluminium never freezes. And here’s the remarkable thing: it succeeded. Save for when the cells were being rebuilt and upgraded a few decades ago, the plant has never been shut down - not since 1927.
This is, in other words, an extraordinary work of human ingenuity. There are other “diversion” schemes, as they’re known, which pass water from one watershed to another. There’s the Snowy Mountains scheme in Australia as well as the Fjarðaál Aluminium Smelter in Iceland and the Kitimat Smelter in Canada. But none have been running as long as the one in the Scottish Highlands.


It’s a remarkable place, amid breathtaking scenery. And, most remarkable of all - even as much of British industry has cratered in recent decades, this place has survived. It survived bombing raids from the Luftwaffe in WWII (the site was a target for the German air force since it produced 80% of the aluminium in British spitfires). It survived energy price shocks (mostly because it has its own supply of power and doesn’t have to rely on fuels imported from elsewhere). It survived the onslaught of cheap Chinese competition, which took down most of its counterparts. Now, however, it faces a new threat, the imposition of tariffs by the United States.
I’ve written in more depth about this for Sky News. Do, if you have any interest, have a read of that piece. And do, if you find yourself in Fort William, go have a look at the Lochaber plant - you can see those pipes coming down from the mountain from far away. They are the end point of an extraordinary network of tunnels that serve a crucial industrial purpose - for the time being at least.





Wow what an incredibly engaging article.
Totally engrossed in that and in 60 seconds I now know so much more about alumnium production. Excellent
Very good. If you ever decide to write a sequel to Material World featuring all the materials you almost covered (wood, cobalt, aluminum), I would buy it in an instant.