The Salt Diaspora
We mostly only pay attention to the chemicals sector when something goes wrong. That's a mistake.
A few weeks ago a freight train carrying (among other things) a cargo of critical chemicals derailed in the town of East Palestine, Ohio. A cocktail of toxic substances including vinyl chloride and butyl acrylate was spilt. A couple of days later, state authorities evacuated the town and “vented” some of the chemicals. Or, to translate into English: they set them on fire.
You’ve probably seen the subsequent pictures: the flames reaching over the streets, the shocking black cloud shadowing above the town like an apocalyptic portent. You’ve probably heard about the fears of local residents about toxic residue, not to mention their frustration that the safety of this town in a poor corner of America is being overlooked by Washington.
But one less remarked on aspect of the story is the background story of the substances inside those railroad carriages. For they represent an invisible part of the modern world, one we tend to ignore 99.9 per cent of the time. Until, that is, something goes wrong.
The chemicals sector is an immensely complex and immensely important part of the modern economy. It will feature prominently in Material World because, controversial as it is, it represents part of the foundations of civilisation. Pretty much everything you can buy features some of the sector’s wares somewhere inside it - or relies on them for their production. Sometimes the reliance is evident if you look hard enough: you spot the plastics inside or outside your phone. But all too often they are completely invisible.
For instance, without the panoply of substances churned out of chemicals works and sometimes carried aboard trains like the one that derailed, we would be unable to refine many of our metals, unable to make the silicon wafers we turn into solar panels and silicon chips. Companies like TSMC would be unable to produce the processors that make your iPhone work without a smorgasbord of gases and solutions ingested by the machines in their fabs in Taiwan.

Our relationship with this world is, to put it lightly, complex. These materials are widely demonised, yet we will rely on them to help us get to net zero, from the flourocarbons that go into heat pumps to the resins that help us make carbon fibre (which reinforces the critical sections of wind turbines).
We need them more than we appreciate. Yet since these substances are frequently dangerous, and since they can often cause harm (sometimes nearly irreparable harm) when released somewhere into the environment, most of us are understandably wary about what feels like a modern kind of dark magic. Especially since most of us don’t really understand what this stuff is actually made of.
PVC is a really good case in point. Polyvinyl chloride - the end product from the stuff they burnt in Ohio - accounts for about a tenth of all the plastic produced around the world. It mostly ends up in the built environment. The double-glazed windows in your home or your office are probably made of PVC, as are the pipes in your plumbing. And when it comes to hard, rigid plastics, this stuff does a pretty good job.

The biggest problem with PVC, beyond the risk of accidents like the one in Ohio, is that for much of the past few decades we didn’t just make it into pipes and gutters and doors, but other things too. For while PVC is hard and rigid in its native state, it is possible to “plasticise” it - to make it softer so you can bend it and turn it into packaging. But this involves adding so-called plasticisers - small organic modules which change the polymer structure so it’s more bendy. And the chief plasticiser used for much of the 20th century was a breed of molecule called phthalates.
Phthalates are, it turns out, nasty pieces of work. They have a tendency to migrate out of the plastic over time and they leach out into the environment where they can be harmful to both humans and to wildlife. Yet we made millions of tonnes of plasticised PVC and used it for years. This helps explain why some argue that we should ban PVC altogether. They have an important point - though they invariably end up conflating the hard stuff (not so bad) with the soft stuff (very problematic).

But none of this answers the deeper question: why have we ended up making so much PVC? Part of the reason we sought out new uses for PVC - turning a rigid plastic into something softer - is that, well, there was an awful lot of PVC knocking around and only so many pipes and windows to be built. And why was there so much PVC knocking around? Well this comes down to something you only really understand when you dive down into the Material World.
Most plastics are made from petrochemicals - they begin as oil or gas (though in theory you could make them from any sources of carbon and hydrogen - hence why some people are exploring making them from captured atmospheric carbon dioxide). But PVC is a little different. For while part of it does indeed derive from ethylene, a petrochemical from oil refineries, actually 56% of PVC’s weight is something else: chlorine. And where does that chlorine come from? Salt.
That’s right: this chemical, the toxic stuff they were burning in East Palestine, is a prominent part of what you might call the “salt diaspora”, the long chain of different substances we use every day without realising they are derived from the very substance we sprinkle over our chips.
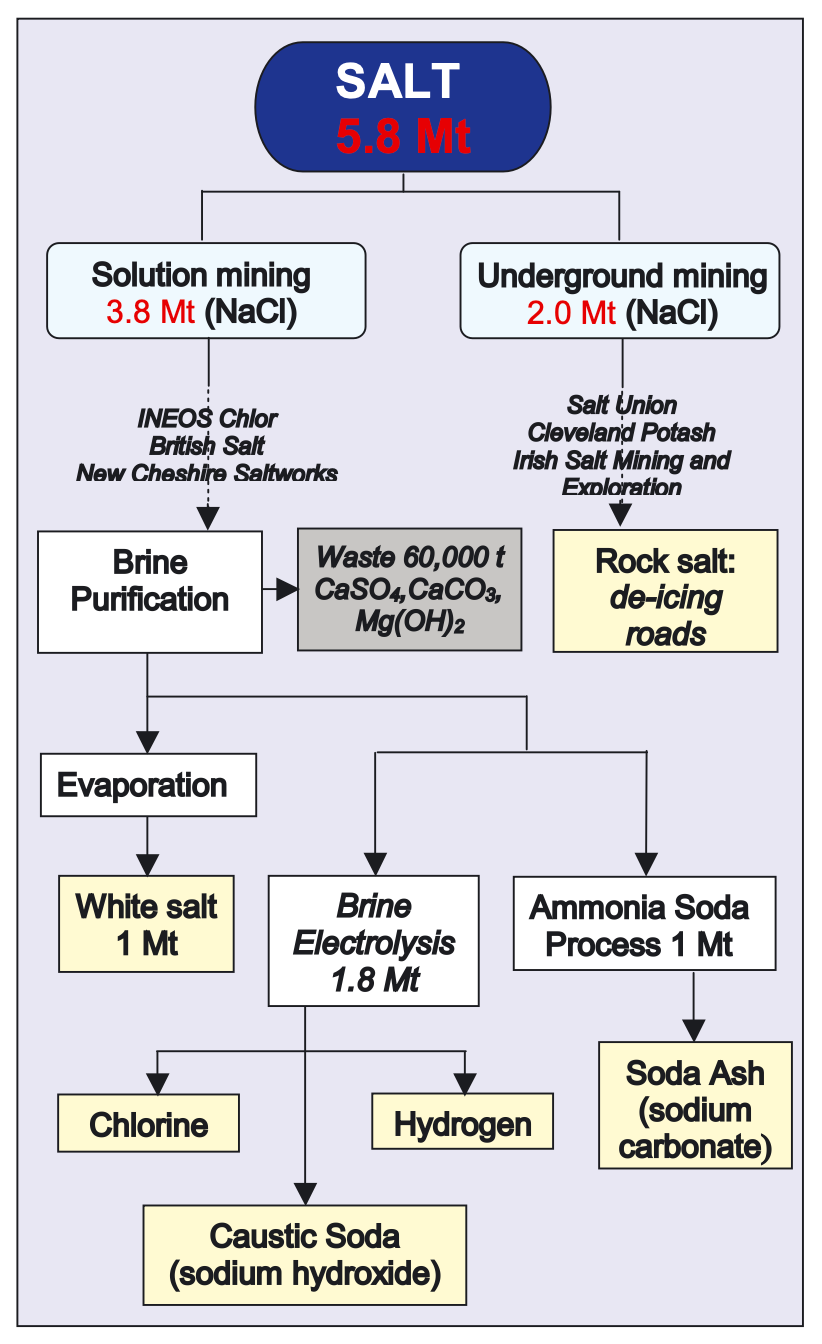
Of the six materials I feature in my forthcoming book, Material World - sand, salt, iron, copper, oil and lithium - salt was probably the one that raised the most eyebrows when I mentioned it to friends and colleagues. How on earth does this commonplace staple merit its place alongside, say, the steel with which we make our cities or the silicon from which we make our semiconductors? The short answer is that salt is about far more than sprinkling on food. Salt remains the bedrock of our chemicals and pharmaceuticals systems.
Indeed, salt for sprinkling on food (“white salt” in the diagram above) is actually one of the smaller end uses these days. Much more of the salt we get out of the ground in this country (we and for that matter the US still get most of our salt from underground deposits - more on that in the book) goes into the chemicals trade. We turn salt into a whole array of substances and solutions we then use to refine metals, to convert into bleaches and into substances like vinyl chloride.
One of the most important of these substances is caustic soda - the bottom-most item on the flow chart above. We use it to make paper, to make detergents, to make textiles and to purify water. We use it to turn lithium into the battery grade materials which will help power an electric car. There’s much more on this in the book but, long story short, caustic soda is, alongside soda ash, one of the great unsung heroes of the modern world. It’s really, really important. And we get it from salt.
The more I wrote about salt, the more it struck me that of all the materials I was investigating, this was arguably the most intriguing. Barely understood, barely appreciated, mostly overlooked, this ancient condiment, which gave its name to the Roman goddess Salus, is still an integral part of the health/pharmaceutical/chemicals system today. As you’ll see when you read Material World, much of the section on salt is about how modern humanity is still tracing out the same salt routes as it did thousands of years ago. Indeed, you could make the case that the train which derailed so disastrously in Ohio was following a 21st century salt route.
And that’s only the start of it, for not only does its story tell us about the modern economy, salt tells us about the nature of power. Salt was used as a tool of diplomacy and as a weapon of war. It was one of the earliest bedrocks for the fiscal system. Salt was power. To some extent, it still is.
But that’s for another day (upon which note please do pre-order a copy of the book - the reason I keep asking is that it really does help, even at this early stage). In the meantime let’s think for a moment about what this all means for PVC. The chemical process to turn salt into caustic soda - sodium hydroxide - also produces a lot of chlorine.
Chlorine is pretty important for purifying water and a few other purposes you can see above but it is, frankly, somewhat less useful than caustic soda. The upshot is that chemicals plants tend to turn out rather more chlorine than they need. You probably see where we’re heading here…
Part of the explanation for why the world has produced quite so much PVC in decades gone by - and part of the reason we ended up turning it into all sorts of nasty plastics we never should have - is that it was seen as a useful way of absorbing a lot of the chlorine produced when we electrolysed salt. Chlorine gas is, as you’ll know, very dangerous. So we need to do something with it. And given we produce rather a lot of it as a side-product when making caustic soda, PVC serves, among other things, as a good way to “soak up” what would otherwise be excess chlorine.
This is, of course, a simplification. I’m sure PVC aficionados will beg to differ. But you get the idea: there’s a strong argument that part of the reason we have quite so much PVC in the world comes back to the chemistry of salt. It comes back to the fact that table salt is sodium chloride and that the second half of the compound needs somewhere to go when we split it apart.
It is, in other words, a consequence of the flow charts much like the ones you see above. When I wrote a while back that we need more maps, this helps underline why: so we can begin to understand the networks of functionality that underpin the modern world. Because PVC and its part in the salt diaspora is only one small dimension of this. Consider the carbon dioxide produced as a byproduct of the Haber Bosch process: we use that for all sorts of purposes which turn out to be really important. But it’s mostly just a by-product of a chemical reaction.
And the more we understand how this interwoven world really functions, the better equipped we will be to do something about all the problems and issues we face. Take plastics. For all that we talk about using less of them, the precise opposite is happening. Long after carbon emissions have peaked, plastic production will continue to mount, according to nearly every projection out there. Here’s one from the OECD:
In much the same way as PVC is only one part of a massive salt diaspora, it is also only one part of a massive plastics diaspora which gets bigger with every decade that goes by. And it does so for the same reason it always has: plastics are very useful and very cheap (especially things like PVC which are simultaneously soaking up the side-product from a slightly more important business). But the more plastics there are, the more risk there will be of that plastic going somewhere it shouldn’t. There will be more chance we misuse it or fail to recycle it. There will be more trains carrying it around like the one that derailed in Ohio.
Just look at some of the chemicals being carried in the train. There was propylene glycol, one of the main inputs in the manufacture of polyester. There was isobutylene, used to make everything from rubber and solvents to resins and chewing gum. There were pellets of propylene, the world’s favourite plastic (which also features prominently in Material World), as well as ethylene glycol monobutyl ether, which we use to make varnishes, agricultural chemicals and cleaners.
Much of this stuff is toxic or at the very least an environmental pollutant. Vinyl chloride can be carcinogenic, and tends to decompose into other harmful compounds. Ethylhexyl acrylate, another of the substances in the accident, can irritate the skin, eyes and respiratory tract. It is clearly not good news when these things get out into the wild. And big questions remain about what’s going on in Ohio.
But the substances represent the tip of a chemical iceberg that supports many of our lives. The task of my book, and these accompanying articles, is to provide an open-eyed look at the Material World upon which we all depend. Ugly, toxic, uncomfortable as it sometimes is, it’s inextricably interwoven with our lives.
This is a free newsletter. Please feel free to share or forward to anyone who you think will be interested. And if you’re interested, do subscribe. Better still, pre-order a copy of Material World, out in the UK in June and in the US in November.








Fascinating read. Thanks! I've preordered in the US, though the cover and subtitle were nothing like what I was expecting from the UK version's page.
Incidentally, what's the difference?
Looking forward to the book.
Best regards, Pol Knops